Key Takeaways from Persistent’s ‘Let’s Talk Pharma’ Roundtable held on January 19, 2024
COVID-19 was a test of endurance and ingenuity for the global pharma industry. The need to deliver a clinically approved vaccine during a raging pandemic stressed the importance of collaboration, agility, and rapid innovation amid lockdowns and disrupted supply chains. It also unearthed critical gaps – primarily data and operational silos – that hindered innovation at speed.
Despite the disruptions, and just a year after it reported the first case, India launched the world’s largest vaccination drive with two vaccines, one of which was the homegrown Covaxin. This was possible only with a concerted effort from the pharma industry, regulators, and allied partners such as healthcare professionals, all helmed by digital processes to speed up clinical trials, approvals, and go-to-market.
The continued stakeholder collaborations and point digital transformations have set the Indian pharma industry on a path to hit $130 billion by the end of this decade. Projections peg the industry growth to overshoot its current rate by three times by 2050, making it essential for pharma companies to double down on technology as a transformation lever across the value chain.
Persistent organized a roundtable with leading industry players to discuss the road ahead and identify possible synergies. Five technology tailwinds emerged. These are:
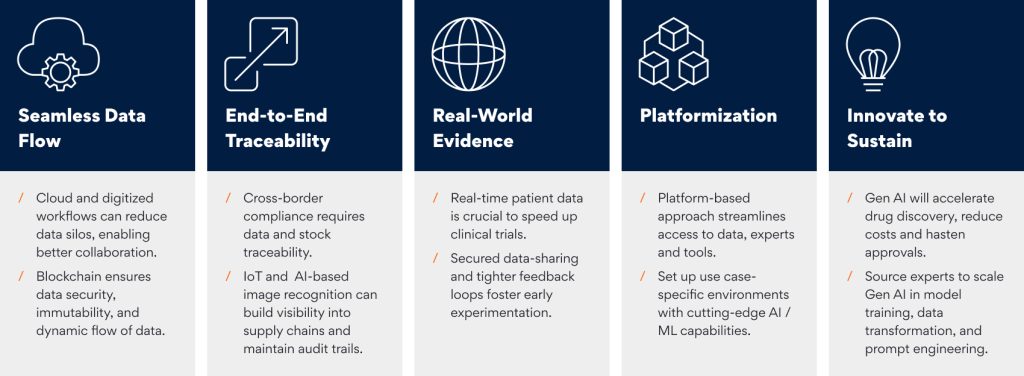
- Seamless Data Flow: The pharma value chain is riddled with data silos that continue to hinder collaboration and prolong turnaround times. Players must digitize workflows, adopt cloud-first infrastructure, and prioritize ground-up security to ensure clinical, patient, and proprietary data can be accessed dynamically and securely. Blockchain offers a compelling use case for dynamic, identity-stamped access while ensuring data immutability, security, and integrity. This will require forging strategic partnerships with technology players with proven expertise in operationalizing multi-source data pipelines, platforms, and analytics undercut by deep domain understanding.
- End-to-end Traceability: For pharma companies looking to scale up globally, cross-border regulatory compliance is crucial. They need granular, point-by-point visibility across the supply chain to maintain product trust and establish compliance. The interoperability of the Internet of Things (IoT), clubbed with artificial intelligence-led optical character/image recognition, can transform distributed supply chains into smarter, connected, and data-driven sites. This will allow granular visibility into cargo movement, track individual batches, and help maintain robust audit trails. Operationalizing IoT across global supply chains requires deeper technology integration and the know-how to glean relevant insights from reams of data. Since IoT is an ecosystem of burgeoning devices and ever-growing network edges, an IT service provider can help pharma companies tap into its potential while proactively recognizing inherent risks and working to mitigate them.
- Real-World Evidence: Digital therapeutics and telemedicine have created avenues to pool real-world data in clinical trials and pharmacovigilance. Scaling this up will require collaborating with players in allied industries, such as healthcare providers, insurance payers, and practitioners, to share time-sensitive patient-critical data. Although this will increase the compliance burden around regulatory mandates on data privacy and personal information (PII, HIPPA), it will also give pharma companies the much-needed growth lift. Creating secured data-sharing mechanisms across the ecosystem will be key, as will the need to create tighter feedback loops that foster experimentation earlier and at cheaper costs.
- Platformization: Access to relevant historical data, experts, and tools can be streamlined through a platform-based approach to operations. This will foster a more homogenous technology adoption across the value chain and help orchestrate necessary checks and balances from data security and regulatory standpoints. The cloud offers a breakthrough here, but before they can get started, pharma companies must partner with cloud transformation leaders who can set up pharma-specific environments that leverage cutting-edge capabilities around machine learning and artificial intelligence to supercharge next-gen pharma companies.
- Innovate to Sustain: Gartner predicts that Generative AI (GenAI) will make 30% of new drug discoveries by as soon as the next year. Technology will reduce costs from discovery to go-to-market and cut down cycle times on regulatory approvals. This heralds a new age in the Indian pharma industry, where players will have to leverage technology as a core lever and not as a mere enabler. Understanding the implications and implementing GenAI at scale will require expertise in model training, data transformation, and domain-aligned prompt engineering.
Reimagine Pharma Value Chains with Persistent
Persistent Systems has invested in innovation to anticipate the needs of the future-ready pharma industry. Our in-house R&D unit, the Persistent Innovation Labs, has created accelerators aligned with the needs of the biopharma industry. Our multi-disciplinary teams have received accolades for their innovative work, most recently the Google Cloud 2023 Social Impact Partner of the Year Award.
With a three-decade-long focus on AI and data, GenAI comes as a natural progression to our expertise that has led to powerful accelerators that can streamline various bio-pharma functions. These AI-powered accelerators have been deployed in diverse customer projects themed on genomics, molecular biology, biomedical imaging, knowledge graphs, drug design and discovery, drug repurposing, vaccine design, cell and gene therapy, biomarker-guided diagnostics development, clinical research, patient personalization, public health management and real-world evidence.
Our close partnerships with leading hyperscalers (AWS, Google Cloud Platform, Microsoft) allow us to innovate faster with GenAI capabilities. These partnerships open our way for early access to GenAI tools to build in-house accelerators with industry-relevant use cases. This pre-market access fosters co-development partnerships with hyperscalers for specific customer use cases. In addition, in specific scenarios, these hyperscalers also provide funding support, organize joint events and create thought leadership publications based on Persistent initiatives.
To revolutionize your pharma business with technology, get in touch with us today.








