The healthcare industry is increasingly getting more focused on outcomes. To demonstrate drug and treatment value, life sciences companies are looking at Real World Evidence (RWE) to complement Randomized Clinical Trials (RCTs). FDA defines Real-world evidence as the clinical evidence regarding the usage and potential benefits or risks of a medical product derived from analysis of real-world data (RWD). Some sources of RWD are shown in Figure 1. The industry is using real-world evidence (RWE) for multiple use cases – vaccine development, drug development, and digital therapeutics beyond traditional safety-related applications. Couple this with increasing support from regulators and the prevalence of technologies to analyze and devices to capture data digitally. We see that RWE will continue to increase its usefulness at multiple stages of therapy development.
Regulatory acceptance of RWE has been very encouraging. In November 2018, FDA released an open-source app – my studies, to enable the collection of RWE via patients’ mobile devices.
More recently, in September 2021, FDA released the latest draft guidance on RWE “Real-World Data: Assessing Electronic Health Records and Medical Claims Data to Support Regulatory Decision-Making for Drug and Biological Products.” It signaled to companies that RWE could be used in regulatory approvals. Outside the US, the UK has also made the CPRD database of EMR data open to all. 80% of pharma companies purchase access to this data now.

Impact of RWE Analytics in Healthcare
RWE analytics can play an essential role in helping life sciences companies make a case to regulators in demonstrating the need for new treatment. Precision medicine is gaining traction and increasingly filling clinical development pipelines. RWE can identify patient segments with unaddressed needs and help demonstrate the status of a drug as an orphan drug. Targeting drugs to specific patient populations allows for improved treatments and reduced side effects.
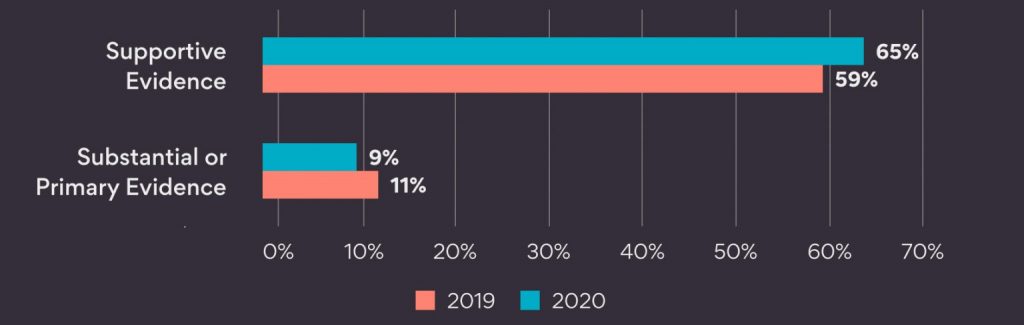
By assisting with treatment valuation, RWE analytics can play a critical role in obtaining fair reimbursement from payer organizations. Me-too compounds are increasing in the pharmaceutical industry. Me-too compounds are similar to pre-existing drugs but have minor modifications. This leads to a fierce competition for market share. RWE can serve to differentiate a treatment from the crowd.
Life sciences companies can improve the clinical study design of postmarket studies by leveraging RWE analytics. Traditionally, regulatory approvals of new drugs are based on randomized clinical trials (RCTs). RCTs mandate an eligibility criterion for patient inclusion. It ensures homogeneity in the patient group and representativeness in the findings. However, this comes at the cost of excluding specific patient subsets – such as those with comorbidities. RWE analytics can be beneficial in refining patient subpopulations and study endpoints. It can support conclusions about therapeutic products on treatment effectiveness and safety profile. RWE provides real-world insights on patients with comorbidities who may have been excluded at the clinical trial stage.

The insights provided by RWD can only be as good as the analytics platform mining the data. Hence, choosing the right partner with extensive healthcare domain experience as well as analytics expertise becomes crucial.
Persistent’s ‘Patient Care NXT’ Platform
Persistent’s ‘Patient Care NXT’ Platform can help organizations build a powerful RWE analytics platform faster. It’ is powered by Microsoft Cloud for Healthcare. It enables healthcare providers and pharma companies to improve patient outcomes. It ensures patient-centered care by harnessing the power of the Microsoft ecosystem, Power Platform, and Azure.
Patient Care NXT is built on Persistent’s unparalleled experience delivering digital healthcare solutions, including:
- 15+ years of broad healthcare industry experience and understanding
- 20+ years of large-scale, complex integration and development experience for global organizations
- A award-winning track record of providing “exceptional customer-centricity in the delivery of core technologies across all geographies” in the healthcare industry.
- Exceptional Delivery Excellence
- Deep Clinical Expertise
Learn more about Persistent’s ‘Patient Care NXT’ Platform.
Learn more about Persistent’s global Microsoft Practice.






